Spectrophotometry
This linear relationship that exists between concentration and absorbance is the basis for the Beers-Lambert Law.
A = ebc
where e is the molar absorptivity (M-1cm-1) and is the characteristic of the substance as it relates to the amount of light being absorbed at a specific wavelength. 'b' (cm) is the cell path length, and 'c' (M) is the solution concentration.
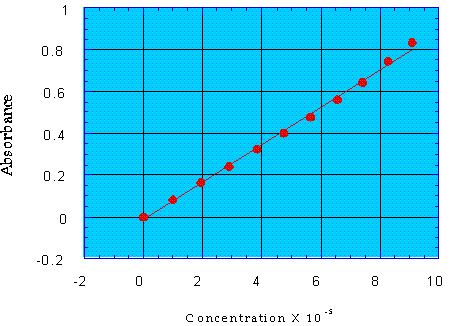
One of the most useful applications of the linearity of the Beer-Lambert Law is in the preparation and use of a calibration curve. A calibration curve consists of a plot of absorption values for varying concentrations of standard solutions.
Once the calibration curve has been plotted, the absorbance of the unknown solution is measured and its concentration determined based on the curve provided by the solutions of known concentration.
 |
![]()