Titration
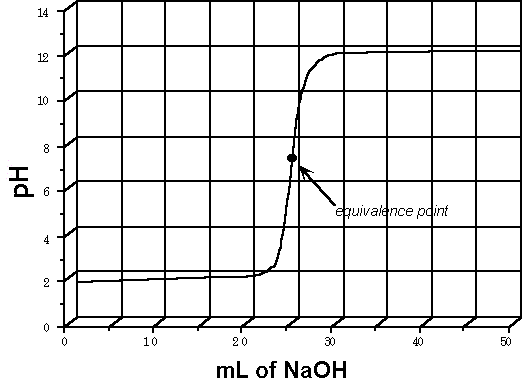
The most common method for determining the pH at the equivalence point is to prepare an acid-base titration curve for the acid-base reaction. Figure 1 illustrates an acid-base titration curve for the titration of 0.100 M HCl (a strong acid), with NaOH (a strong base).

Figure 1. Acid-base titration curve for the titration of a strong acid with a strong base.
Initially, as NaOH is added to the HCl, there is a gradual increase in the pH. However, as you approach the equivalence point, a sharp increase in the pH is observed. Figure 1 shows that the increase in pH covers the approximate range of 2-12 pH units. Therefore, an indicator for this acid-base titration should be sensitive (change color) in the pH range of 2-12. Consequently, any of the indicators listed in Table 1 would be adequate for this titration.
![]()