In a process akin to belling an infinitesimal cat, scientists have managed to tag a protein that regulates the neurotransmitter serotonin with tiny fluorescent beads, allowing them to track the movements of single molecules for the first time.
The capability, which took nearly a decade to achieve, makes it possible to study the dynamics of serotonin regulation at a new level of detail, which is important because of the key role that serotonin plays in the regulation of mood, appetite and sleep.
The achievement was reported by an interdisciplinary team of Vanderbilt scientists in the June 27 issue of the Journal of Neuroscience.
Movie showing motion of individual serotonin transporter molecules on the surface of living nerve cells for the first time. The molecules are tagged with fluorescent quantum dots that show up as red points of light. (Jerry Chang / Vanderbilt)
The regulatory protein that the scientists successfully tagged is known as the serotonin transporter. This is a protein that extends through the membrane that forms the nerve’s outer surface and acts like a nano-sized vacuum cleaner that sucks serotonin molecules into the cell body and away from serotonin target receptors on other cells. In this fashion it helps regulate the concentration of serotonin in the area around the cell. Serotonin transporters are an important research subject because they are the target for the most common drugs used to treat depression, including Prozac, Paxil and Lexapro.

“If you are interested in mental health, then serotonin transporters are an ideal subject,” said Sandra Rosenthal, the Jack and Pamela Egan Chair of Chemistry, who directed the study with Randy Blakely, the Allan D. Bass Professor of Pharmacology and Psychiatry.
Problems with serotonin transporter regulation have also been implicated in autism. Two years ago, Blakely and geneticist James Sutcliffe, associate professor of molecular physiology and biophysics, reported the discovery of multiple changes in the serotonin transporter protein that cause the transporter to become “overactive” in subjects with autism. Recently, Blakely and Assistant Professor of Psychiatry Jeremy Veenstra-VanderWeele reported that mice expressing one of these high-functioning transporters exhibit multiple behavioral changes that resemble changes seen in kids with autism.

The brain’s other key neurotransmitters have their own transporter proteins, so scientists can use the capability to track the motion of individual transporter molecules to determine how they are regulated as well.
Attempts to understand how these transporters work have been limited by the difficulty of studying their dynamic behavior. “In the past, we have been limited to snapshots that show the location of transporter molecules at a specific time,” said chemistry graduate student Jerry Chang, who developed the tagging technique. “Now we can follow their motion on the surface of cells in real time and see how their movements relate to serotonin uptake activity.”
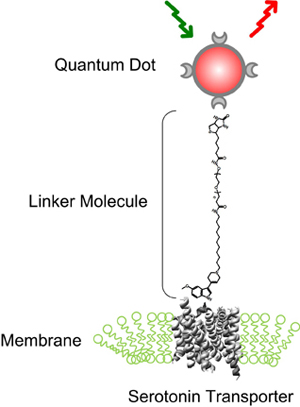
The fluorescent tags that the researchers used are nanoscale beads called quantum dots made from a mixture of cadmium and selenium. These beads are only slightly bigger than the proteins they are tagging: You would have to string 10,000 together to span the width of a human hair.
Quantum dots emit colored light when illuminated and have the useful property that small changes in their size cause them to glow in different colors. Team member Ian D. Tomlinson, assistant research professor of chemistry, developed a special molecular string that attaches to the quantum dot at one end and, on the other end, attaches to a drug derivative that binds exclusively with the serotonin transporter. When a mixture that contains these quantum dots is incubated with cultured nerve cells, the drug attaches to the transporter. As the protein moves around, it drags the quantum dot behind it like a child holding a balloon on a string. When the area is illuminated, the quantum dots show up in a microscope as colored points of light.

“Until now, neurobiologists have had to rely on extremely low resolution approaches where it takes the signals coming from thousands to millions of molecules to be detected,” said Blakely, “We really had no idea exactly what we were going to see.”
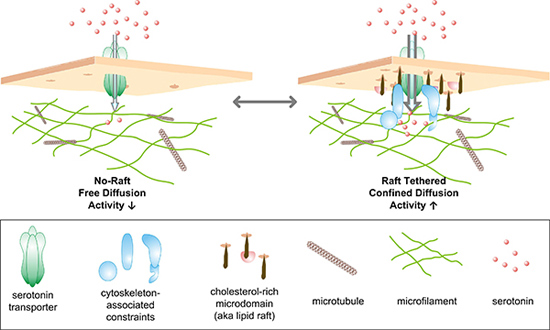
Putting their new procedure to use, the researchers looked at extensions of the nerve cell that are involved in secreting serotonin on the presumption that transporters would be localized there as well. From previous research, the investigators suspected that the transporters would be concentrated in cholesterol-rich parts of these extensions, termed rafts, although the level of resolution with standard approaches was inadequate to provide any clues as to what they were doing there.
The quantum dot studies demonstrated that there were two distinct populations of transporters in these areas: Those that can travel freely around the membrane and those that act as if they are unable to move. They found that the immobile transporters were located in the rafts. When they stimulated the cell to increase transporter activity, they were surprised at what happened. “We found that the transporters in the rafts began to move much faster whereas the motion of the other population didn’t change at all,” Rosenthal reported. Since the mobilized transporters do not leave the rafts, they appear to whizz around inside a confined compartment, as if released from chains that normally keep them subdued. These observations suggest it is likely that the two populations are controlled by different regulatory pathways.
“Now that we can watch transporter regulation actually happening, we should be able to figure out the identity of the anchoring proteins and the signals these proteins respond to that permit transporters to switch back and forth between low and high activity levels,” said Blakely.
“Currently, antidepressant drugs must fully shut down the brain’s serotonin transporters to achieve a clinical benefit,” the pharmacologist said. Such a manipulation can produce a number of unpleasant side-effects, such as nausea, weight gain, sexual problems, fatigue and drowsiness.
“By understanding the basic mechanisms that naturally turn serotonin transporter activity up and down, maybe we can develop medications that produce milder side-effects and have even greater efficacy,” he said. “Our sights are also focused on transferring what we have learned with normal serotonin transporters to an understanding of the hyperactive transporters we have found in kids with autism.”
Other members of the research team include chemistry graduate student Michael Warnement; Ana Carneiro, assistant professor in pharmacology; graduate student Alessandro Ustione; and David Piston, the Louise B. McGavock Professor from molecular physiology and biophysics.
The research was supported by grants from the National Institutes of Health, the Vanderbilt Institute of Nanoscale Science and Engineering and the NIMH Silvio O. Conte Center for Basic Neuroscience Research. Microscopy support was provided by Vanderbilt’s Cell Imaging Shared Resource.