Dark, Heather E.; Shafer, Andrea T.; Cordon, Jenifer; An, Yang; Lewis, Alexandria; Moghekar, Abhay; Landman, Bennett; Resnick, Susan M.; Walker, Keenan A. “Association of Plasma Biomarkers of Alzheimer Disease and Neurodegeneration With Longitudinal Intra-Network Functional Brain Connectivity.” Neurology, vol. 104, no. 4, 2025, e210271, https://doi.org/10.1212/WNL.0000000000210271.
Alzheimer’s disease (AD) is characterized by the buildup of β-amyloid (Aβ), tau, and neurodegeneration, which negatively impact cognitive function by disrupting brain networks. While the effects of cortical Aβ and tau on brain connectivity are known, it is unclear whether plasma biomarkers of AD pathology are related to these changes. In this study, we explored whether plasma biomarkers related to AD (Aβ42/40, phosphorylated tau [pTau-181]), astrogliosis (glial fibrillary acidic protein [GFAP]), and neuronal injury (neurofilament light chain [NfL]) are associated with changes in brain network connectivity over time, and whether these changes are linked to cognitive decline.
We measured plasma biomarkers using advanced assays and collected functional brain network data through resting-state fMRI from cognitively healthy adults in the Baltimore Longitudinal Study of Aging. We also assessed cognitive performance at each scan. Using statistical models, we investigated how these biomarkers related to changes in connectivity, whether these relationships varied by amyloid status, and whether changes in connectivity were connected to cognitive decline.
Our findings (n = 486; average age = 65.5 years) revealed that higher levels of GFAP at baseline were linked to faster declines in connectivity in several brain networks, including somatomotor, limbic, and frontoparietal networks. We also found that the relationship between certain biomarkers, like NfL, and network connectivity changes was stronger in amyloid-positive individuals. Additionally, among a subset of participants with multiple scans, changes in connectivity were associated with cognitive decline, although these results did not hold after correcting for multiple comparisons.
This study suggests that plasma biomarkers of amyloidosis, astrogliosis, and neuronal injury are associated with declines in brain network connectivity, particularly in amyloid-positive individuals. However, the study had limitations, including the absence of certain tau biomarkers (pTau-217 and pTau-231) and comparative PET data.
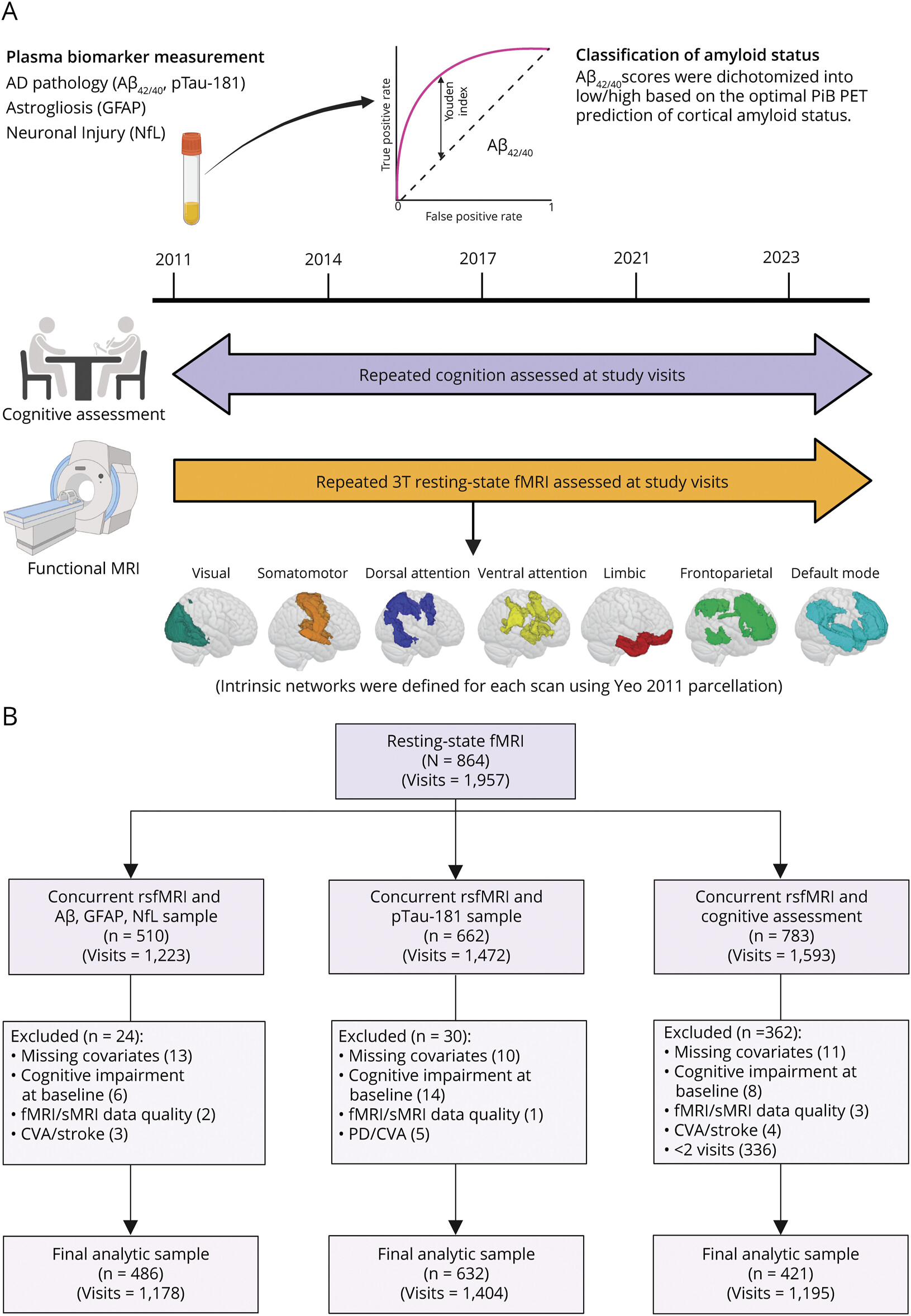
Figure 1 Study Time Line and Study Exclusion Flowchart
(A) BLSA 3T resting-state fMRI scans were initiated in 2011. Baseline visits were considered the first visit for which participants had concurrent plasma biomarker measurement and 3T fMRI. From the baseline biomarker measurement, amyloid status (low vs high) was defined using an ROC analysis of Aβ42/40 for optimal prediction of amyloid (PiB)-PET positivity in BLSA participants (n = 212). Cognitive assessments began before 2011, but only those with concurrent resting-state fMRI scans were included. Finally, using a predefined cortical parcellation, 7 intrinsic functional networks were derived for each resting-state fMRI scan. (B) Study flow diagram for participant selection. Aβ = β-amyloid; BLSA = Baltimore Longitudinal Study of Aging; CVA = cerebral vascular accident; GFAP = glial fibrillary acidic protein; NfL = neurofilament light chain; PiB = 11C-Pittsburgh compound-B; PD = Parkinson disease; pTau-181 = tau phosphorylated at threonine-181; ROC = receiver operating characteristic; sMRI = structural MRI. Created in BioRender. Dark H (2024) BioRender.com/b73m120.