Kjar, A.; Haschert, M.R.; Zepeda, J.C.; Simmons, A.J.; Yates, A.; Chavarria, D.; Fernandez, M.; Robertson, G.; Abdulrahman, A.M.; Kim, H.; Marguerite, N.T.; Moen, R.K.; Drake, L.E.; Curry, C.W.; O’Grady, B.J.; Gama, V.; Lau, K.S.; Grueter, B.; Brunger, J.M.; Lippmann, E.S. “Biofunctionalized gelatin hydrogels support development and maturation of iPSC-derived cortical organoids.” Cell Reports, Volume 43, Issue 11, 2024, Article 114874, DOI: 10.1016/j.celrep.2024.114874.
Human neural organoids are used to study brain biology, but making them more accurate in representing different types of brain cells is still a challenge. In this study, the researchers compared two materials for growing these organoids: Matrigel (a common material) and a new material called GelMA-Cad, which is made from a special gel that helps guide cell development. They found that the GelMA-Cad material helped the organoids develop more like human fetal brain tissue and produced neurons that were more active compared to those grown in Matrigel.
These findings suggest that GelMA-Cad could be a better material for growing neural organoids, allowing researchers to control how the cells develop more precisely. It also offers a simpler and more reliable alternative to Matrigel for research on brain development.
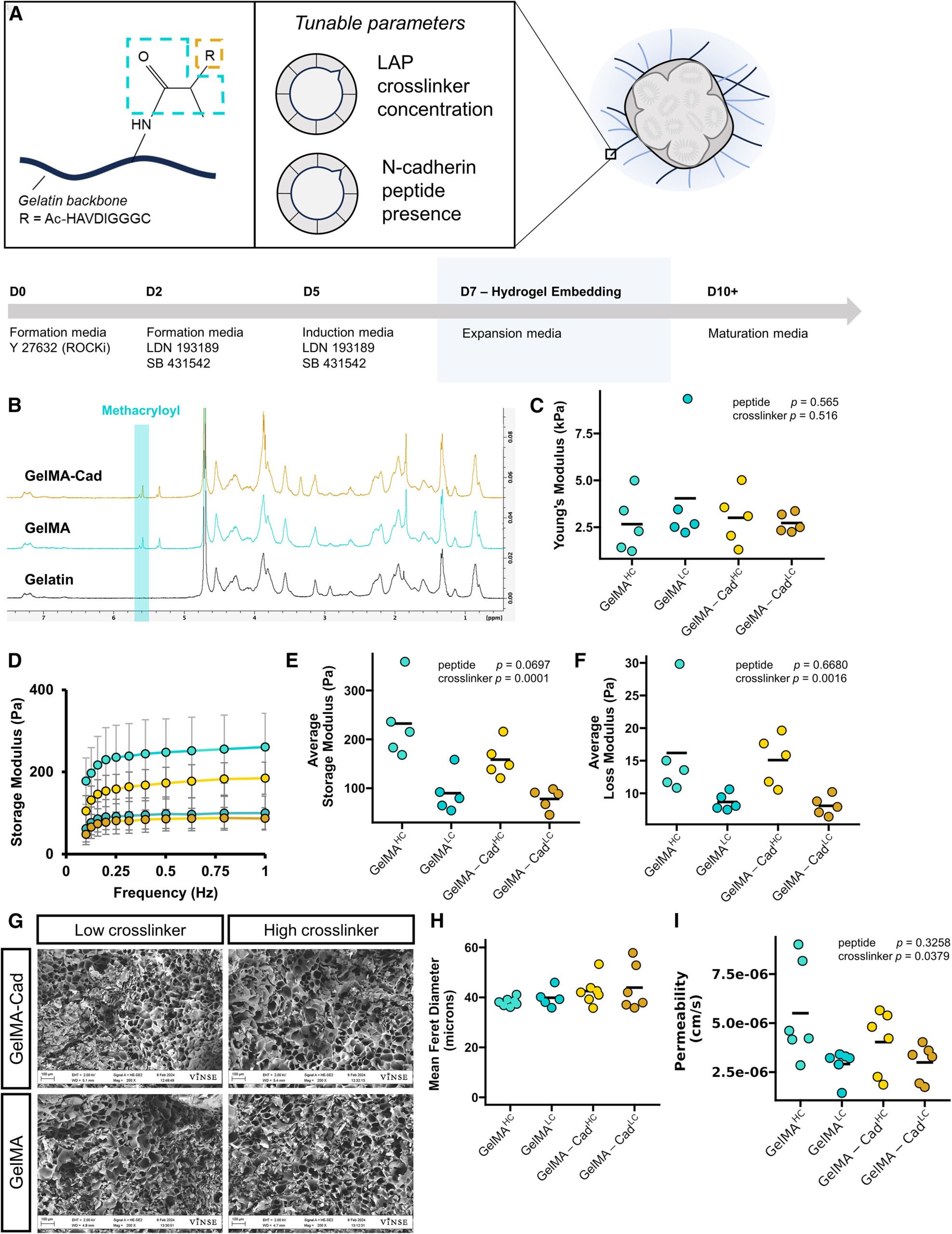
Figure 1. Functionalized gelatin matrix parameters for cortical organoid culture
(A) Schematic of GelMA-Cad, major tunable parameters, and associated organoid culture timeline. GelMA-Cad utilizes a gelatin-based backbone, with a conjugated methacryloyl group (blue), allowing photo-initiated crosslinking. The methacryloyl can be further modified by the addition of an N-cadherin (Cad) peptide mimetic (orange). Adjusted parameters include the LAP crosslinker concentration and N-cadherin peptide presence.
(B) Representative 1H-NMR spectra of gelatin, GelMA, and GelMA-Cad displaying the characteristic peaks associated with methacryloyl.
(C) Atomic force microscope characterization of Young’s modulus. N = 5 measurements, each the average of 512 technical replicates. Bars represent data mean. Statistical significance was calculated with a two-way ANOVA, modeled on crosslinker concentration and peptide presence.
(D) Average storage modulus traces from GelMA and GelMA-Cad hydrogels. N = 5 measurements, error bars represent the standard deviation.
(E) Rheological characterization of average storage modulus across tested frequencies. N = 5 measurements, bar represents data mean. Statistical significance was calculated with a two-way ANOVA, modeled on crosslinker concentration and peptide presence.
(F) Rheological characterization of average loss modulus across tested frequencies. N = 5 measurements, bar represents data mean. Statistical significance was calculated with a two-way ANOVA, modeled on crosslinker concentration and peptide presence.
(G) Representative scanning electron micrographs of lyophilized, crosslinked hydrogels. Scale bar: 100 μm.
(H) Quantification of pore size from scanning electron micrographs. Each point is the mean of measurements made from a single image, N = 6–7 images per condition, each image from a separate preparation. Bars represent data mean.
(I) Apparent permeability coefficient of 3 kDa FITC-dextran in each hydrogel. N = 6 measurements, bar represents data mean. Statistical significance was calculated with a two-way ANOVA, modeled on crosslinker concentration and peptide presence.