Next Event
Which IRB reviews my research during the Second Phase Launch? (July 1, 2025 - December 31, 2025)
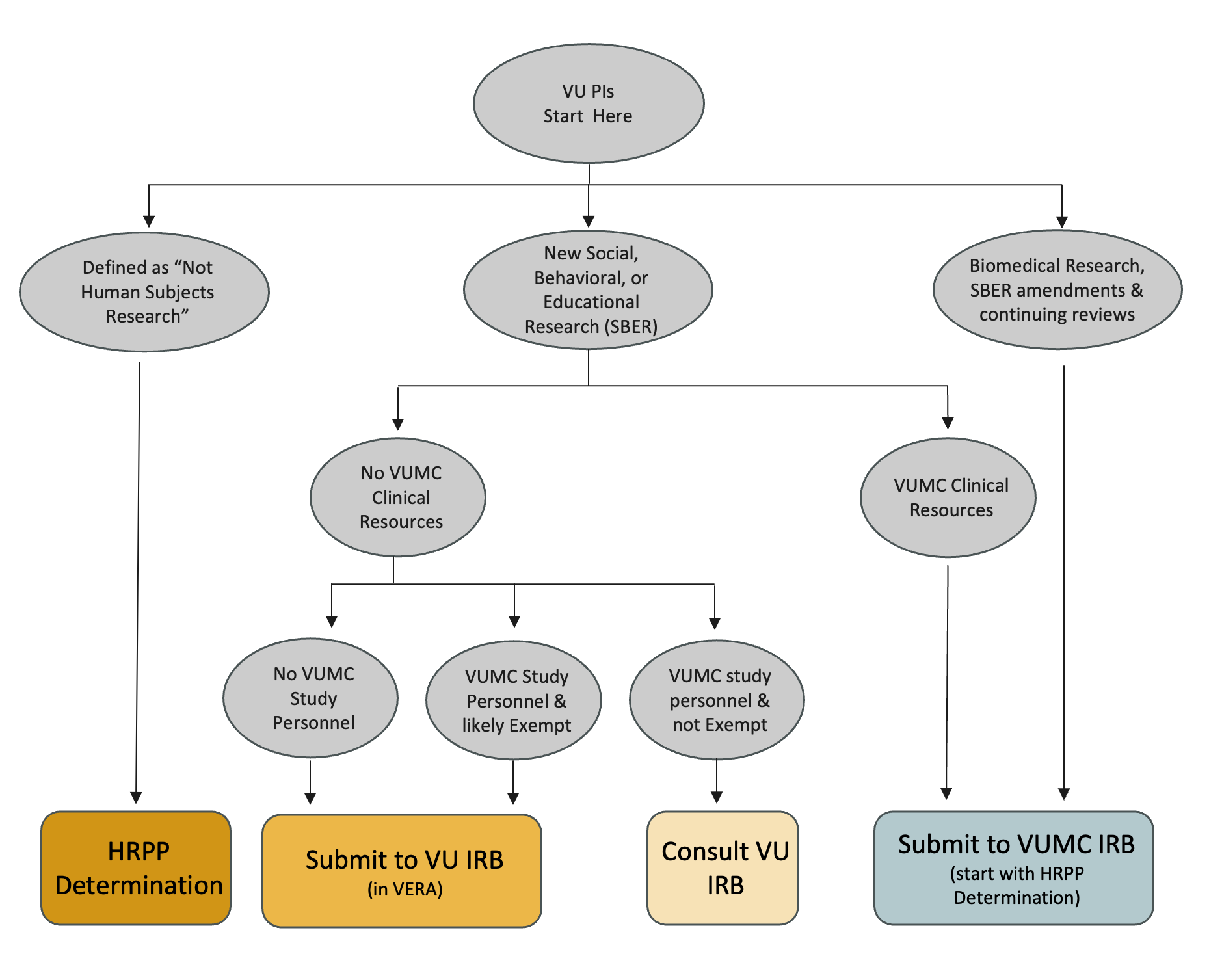
See descriptions of the flowchart categories. VU PIs submitting to the VUMC IRB must include the HRPP Determination with their study material.
