Researchers hope insights into low-light vision of Antarctic icefish can promote better understanding of human health
By Tatum Lyles Flick, Evolutionary Studies communications volunteer consultant
Though many researchers have considered how fish survive in extreme cold, using everything from antifreeze glycoproteins that protect cells to not producing hemoglobin, few have taken a molecular approach to evaluate how they are able to see in such conditions.
In “Adaptation of Antarctic Icefish Vision to Extreme Environments,” published by assistant professor of biological sciences and ophthalmology Gianni Castiglione and others in Molecular Biology and Evolution, Castiglione and his colleagues delve into how Cryonotothenioidea, or Antarctic icefishes, see in very cold, low-light environments.

“These fishes live in temperatures near the freezing point of saltwater (-1.9°C),” Castiglione said, adding that in the Antarctic, snow and ice shift light to redder wavelengths, while also reducing the amount of light that enters the ocean, leading to darker, relatively redder surroundings.
Using likelihood models and ancestral reconstruction, Castiglione and the research team found that, over evolutionary time, Crynotothenioidea experienced genetic mutations, leading to an adjustment in amino acids incorporated into rhodopsin, a protein in the eye’s rod photoreceptors that mediates low-light vision.
“Cryonotothenioidea are so well adapted to subzero temperatures that they dominate 95 percent of biomass surrounding the Antarctic continent,” Castiglione said. “They live across a broad depth spectrum (0-3000 meters) uncommon in other deep-sea fauna assemblages.”
In general, fish who can see in low-light Antarctic waters are able to do so only up to about 60 meters. In addition to the mutations which increase their ability to see, Cryonotothenioidea also have specialized retina that are better able to sense available light.
“These mutations also lower the activation energy associated with retinal release of the light activated RH1, and accelerate its return to the dark-state likely compensating for a cold-induced decrease in kinetic rates,” Castiglione said.
This means that the organism’s eyes are likely more sensitive and it takes less light to start the chemical chain reaction that allows fish to see in such conditions than it would in fish without the genetic mutation. It also allows their eyes to adjust to different levels of light more quickly.
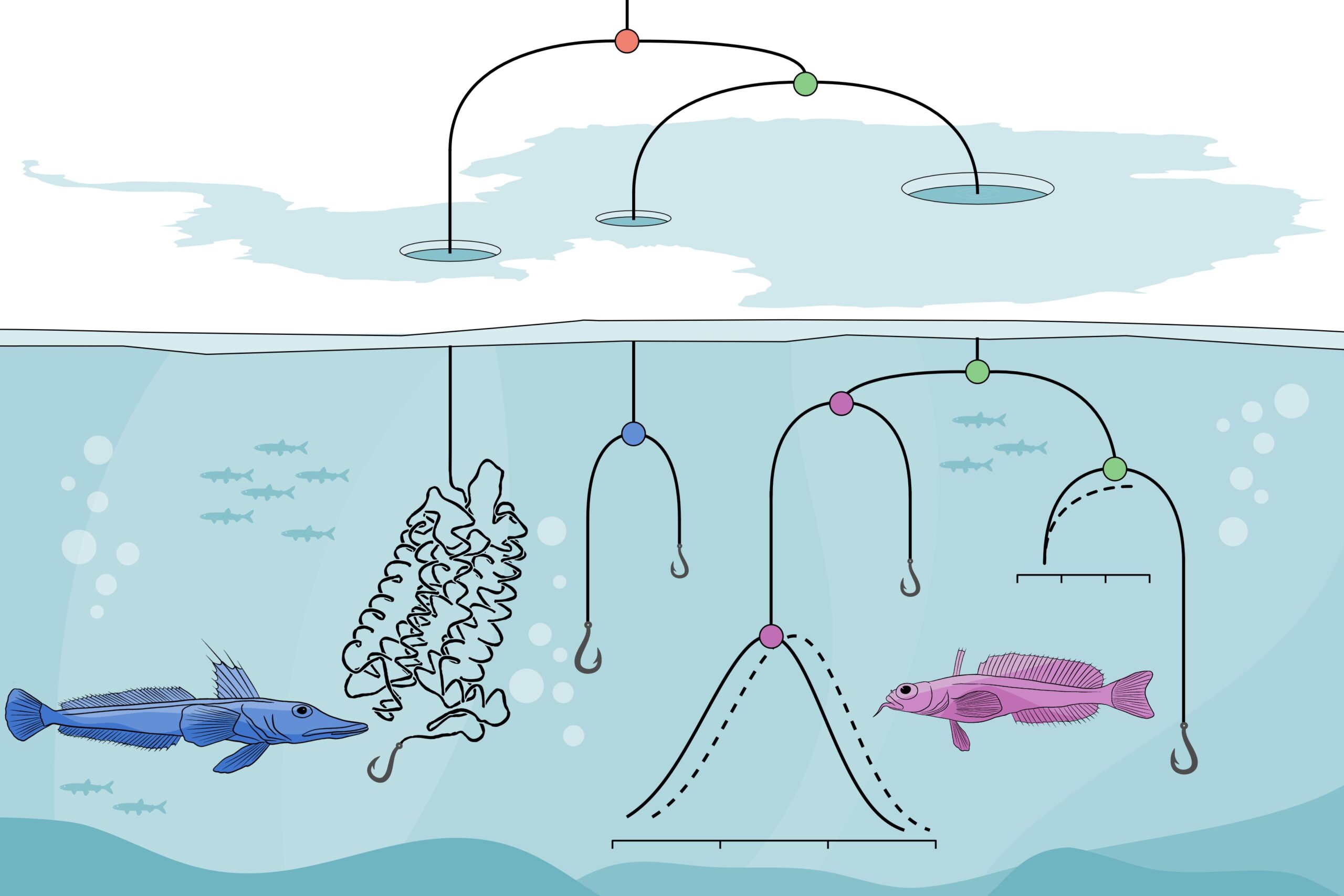
Castiglione’s team found that some of these mutations were “introduced before (S160A) and during (V259M) the onset of modern polar conditions.”
In addition to building a better understanding of how ice fish evolved to successfully thrive in their environment, Castiglione hopes this research can help scientists better understand human diseases.
Castiglione compared these evolutionary adjustments to the selection of less viscous engine fluids in colder climates, to accommodate the temperature’s effect on a fluid’s ability to move.
“The principle is the same at the molecular level of DNA and proteins,” he said. “An example that was known previously is that enzymes (such as those involved in energy production) adapt to cold temperatures by becoming extremely flexible, which compensates for the ‘slowing-down’ effects of cold temperatures. This flexibility makes them very unstable in normal warm temperatures, but ‘just-right’ in cold temperatures.”
The same idea applies to proteins in the eye and can help us better understand mutations that may cause harm to human health.
“This is important because we are warm-blooded, and so destabilization of proteins has no benefit, and has in fact, very negative, disease-associated consequences, such as blindness in the case of rhodopsin destabilization,” Castiglione explained. “It is akin to using Canadian winter engine fluids during the summer in Tennessee.”
Castiglione and his team believe these evolutionary adjustments in rhodopsin offer a unique way to assess and understand how species change over time at the molecular level to survive.
“We also hope it further raises the importance of rhodopsin stability in mediating adaptation of the visual system. In the long term, we hope that icefishes will be increasingly leveraged to understand human disease, since they form an extreme contrast to our biology that we believe is mechanistically informative.”
With climate change on the horizon, the future for ice fishes, however, may not be so bright.
“As sea-ice continues to melt, it will change the light and temperature of the icefish environment, forcing the icefish to very likely find themselves at an evolutionary ‘mismatch’ between their environment and their genetics,” he said. “Over evolutionary time, we suspect they will adapt by blue-shifting their vision to match the lack of sea-ice (which red-shifts light), and we suspect that the kinetics of rhodopsin may slow down again, as cold temperatures warm up and the fast kinetic compensation is no longer required (but in fact deleterious).”
While only a couple of mutations helped these fish see better in low light, other adaptations that allow them to survive in the Antarctic occurred from many more genetic alterations and may not be easy to overcome.
“As oxygen levels decrease due to warming Antarctic waters, it will not be “easy” to re-evolve hemoglobin, as these genomic regions are now pseudogenes that have been accumulating mutations for millions of years due to a lack of purifying selection.”
This paper was selected as an editor’s highlight and featured as the cover image of the journal.
Funding Statement: This work was supported by a National Sciences and Engineering Research Council (NSERC) Discovery grant (BSWC) and Vision Science Research Program Scholarships (GMC, FEH, AVN). The 11-cis-retinal was generously provided by Rosalie Crouch (Medical University of South Carolina).
Citation: Castiglione, G.M.; Hauser, F.E; Van Nynatten, A; Chang, BSW. “Adaptation of Antarctic Icefish Vision to Extreme Environments.” Molecular Biology and Evolution (2023): msad030. Link
