![Medicine - Cancer Biology E-Newsletter [Vanderbilt University]](https://cdn.vanderbilt.edu/vu-URL/wp-content/uploads/sites/119/2017/11/19151037/cancer-biology-design-004.png)
|
|
August 2024
|

The new academic year has arrived, and while it still feels like summer outside, the fall semester is underway! Our second-year students have begun Cancer Biology classes and third-year students are in the midst of Phase II Qualifying Exams. Please wish them luck (and bring them coffee)! As you will see highlighted in the newsletter, all of our students are hard at work, presenting at conferences, earning awards and fellowships, publishing manuscripts, and contributing to the field of cancer research. I am so proud of their accomplishments.
The Annual Retreat for the Program in Cancer Biology at the Hermitage Golf Club, organized by our diligent Cancer Biology Student Association, is just around the corner on September 12! Please make every effort to attend. It will be a fun and exciting day of sharing cancer research!
Science Hour will also resume in mid-late September. Please be on the lookout for those announcements.
I hope you all have a safe and happy September!
Take care,
Rachelle Johnson, PhD
Program Director/DGS
Program in Cancer Biology Research and News
Third-year PhD student Déja Grant was recently selected as a Gilliam Fellow in the 2024 cohort. The School of Graduate Studies, Meharry Medical College student is the first Meharrian to receive the award. Each year, only 50 graduate students are selected for this honor!
Howard Hughes Medical Institute (HHMI), the nation’s largest private biomedical research institution, created the Gilliam Fellows Program in 2004 to nurture excellence and diversity in science and in recognition of the importance of mentorship in developing tomorrow’s scientific leaders. Fellows are offered leadership training, professional development and opportunities to engage with and learn from peers, program alumni and HHMI scientists. The program provides $53,000 in annual support for each student’s dissertation research for up to three years.
 Déja is working in the lab of her advisor, Rachelle Johnson, PhD, Associate Professor of Medicine, Program Director/Director of Graduate Studies for Cancer Biology at Vanderbilt University. “The emphasis on the mentor-mentee pair is truly unique,” Dr. Rachelle Johnson said. “I look forward to working with Déja to determine how intracrine signaling pathways disrupt the niche to promote outgrowth of dormant tumor cells in the bone marrow and seeing all that Déja will accomplish through her Gilliam fellowship.” Déja is working in the lab of her advisor, Rachelle Johnson, PhD, Associate Professor of Medicine, Program Director/Director of Graduate Studies for Cancer Biology at Vanderbilt University. “The emphasis on the mentor-mentee pair is truly unique,” Dr. Rachelle Johnson said. “I look forward to working with Déja to determine how intracrine signaling pathways disrupt the niche to promote outgrowth of dormant tumor cells in the bone marrow and seeing all that Déja will accomplish through her Gilliam fellowship.”

Gwen Joseph (Johnson Lab) received her Notice of Award for the NCI F99/K00 Transition Award! She was first selected as the Vanderbilt nominee, as only one student from each institution can apply for this prestigious award before being chosen as one of 24 recipients to receive the funding. The NCI Predoctoral to Postdoctoral Fellow Transition Award (F99/K00) supports outstanding PhD candidates as they complete their dissertation research training (F99 phase) and transition to mentored, cancer-focused postdoctoral career development research positions (K00 phase). The purpose of the NCI Predoctoral to Postdoctoral Fellow Transition Award (F99/K00) is to encourage and retain outstanding graduate students recognized by their institutions for their high potential and strong interest in pursuing careers as independent cancer researchers. Gwen is researching the impact of PD-1 blockade on the skeleton. She is investigating the mechanisms behind why patients treated with immune checkpoint inhibitors (ICIs), such as a-PD-1, develop musculoskeletal toxicities and why these ICIs are ineffective in the bone metastatic setting. This is clinically relevant given the steady increase in patients being treated with ICIs since their FDA approval ~15 years ago, as well as the large number of cancer patients that develop bone metastases. Her data suggests that PD-1 blockade expands effector T cell populations, which are capable of promoting bone resorption and leading to a loss of bone microarchitecture and strength. Increased bone resorption is also known to correlate with the release of pro-tumor growth factors, which could promote tumor growth in the bone microenvironment, which is an aim she hopes to investigate more in her K00-supported postdoctoral training. This NCI F99/K00 Transition Award provides her with an excellent opportunity to continue investigating this project for the duration of her graduate school training and up to 4 years of her postdoctoral training. We are all so proud of you!

Jared Rhodes (Goldenring Lab) has received the National Science Foundation award. Thirty-four Vanderbilt University alumni, students and incoming students have been named National Science Foundation graduate research fellows for 2024. The prestigious fellowship program assists exceptional graduate students pursuing research-based master’s and doctoral degrees across various fields, including science, technology, engineering, mathematics, STEM education and social sciences supported by NSF. Since its inception in 1952, the NSF GRFP has supported graduate students financially through a $37,000 annual stipend and a $16,000 allowance for educational expenses, along with opportunities for professional growth and international research endeavors.
 Congratulations to Reilly G. Fankhauser, MSTP, in the Balko Lab for his spotlight piece on an article in the August issue of Cancer Immunology Research! A recent article by Siddiqui et al. published in Cancer Immunology Research discusses immune checkpoint inhibitor-associated myocarditis (ICI-MC), a rare but potentially life-threatening complication associated with immune checkpoint inhibitors used to treat cancer. Siddiqui and colleagues identified novel cellular subsets and proposed various mechanisms that could contribute to the pathogenesis of ICI-MC. These new insights should help move the field toward developing improved treatment and prevention options, ultimately improving patient outcomes. Dr. Balko and his team (Reilly Fankhauser, Dr. Douglas Johnson, Dr. Javid Moslehi) were invited to write a spotlight piece on this article, highlighting the importance of their results and contextualizing the findings within our current understanding of the pathogenesis of ICI-MC. Read more here. Congratulations to Reilly G. Fankhauser, MSTP, in the Balko Lab for his spotlight piece on an article in the August issue of Cancer Immunology Research! A recent article by Siddiqui et al. published in Cancer Immunology Research discusses immune checkpoint inhibitor-associated myocarditis (ICI-MC), a rare but potentially life-threatening complication associated with immune checkpoint inhibitors used to treat cancer. Siddiqui and colleagues identified novel cellular subsets and proposed various mechanisms that could contribute to the pathogenesis of ICI-MC. These new insights should help move the field toward developing improved treatment and prevention options, ultimately improving patient outcomes. Dr. Balko and his team (Reilly Fankhauser, Dr. Douglas Johnson, Dr. Javid Moslehi) were invited to write a spotlight piece on this article, highlighting the importance of their results and contextualizing the findings within our current understanding of the pathogenesis of ICI-MC. Read more here.
 Kara McNamara (K. Wilson lab) Kara McNamara (K. Wilson lab)
Kara traveled with the ASPIRE on the Road team to Boston! Twelve graduate students and postdoctoral fellows in the Biomedical Research Education and Training’s ASPIRE Program recently visited Boston-area biotechnology and pharmaceutical companies to learn about career paths, hear from key leadership, tour facilities, and meet with alums. They visited companies including MOMA Therapeutics, Sanofi, Merk, and J&J Innovative Medicine. While at these visits, trainees met with employees and management to discuss company structure, hiring processes, and the transition to industry from academia. A happy hour was held so current trainees could meet with past Vanderbilt alumni. It was an incredible trip planned by Ashley Bradley, the assistant dean of biomedical career engagement and strategic partnerships.
|
|
RECENT VANDERBILT FACULTY AND TRAINEES PUBLICATIONS:
Functional overlap of inborn errors of immunity and metabolism genes defines T cell metabolic vulnerabilities. Andrew R Patterson, KayLee K Steiner, Emilie L Fisher, Jeffrey C Rathmell, et al. Sci Immunol. 2024 Aug 16;9(98):eadh0368.
MTGR1 is required to maintain small intestinal stem cell populations. Cell Death & Differentiation. Sarah Short*, Rachel Brown, Jennifer Pilat, Jing Wang, Yash Choksi, Jeremy Goettel, Ken Lau, Christopher S. Williams*, et al. Research Square (preprint)
Chronic High-Salt Diet Activates Tumor-Initiating Stem Cells Leading to Breast Cancer Proliferation. Tucker L, Zent R, Lannigan DA, Rathmell JC, Tiriveedhi V, et al. Cells. 2024 May 25;13(11):912.
HIF-2α expression and metabolic signaling require ACSS2 in clear cell renal cell carcinoma. Bacigalupa ZA, Arner EN, Vlach LM, Wolf MM, Beckermann KE, Rathmell WK, Rathmell JC, et al. J Clin Invest. 2024 Jun 17;134(12):e164249.
Controlling the STING pathway to improve immunotherapy. Wilson JT. Nat Nanotechnol. 2024 Jun;19(6):718-720.
Peripheral T Cell Development and Immunophenotyping of Twins with Heterozygous FOXN1 Mutations. ImmunoHorizons. Kelsey Voss, Todd Bartkowiak, Jonathan Irish, Jeffrey Rathmell, et al. Immunohorizons. 2024 July 1;8(7):492-499.
Unlocking the Next Frontier in Precision Oncology: Addressing Drug-Tolerant Residual Disease. Lin JJ, Gainor JF, Lam VK, Lovly CM. Cancer Discov. 2024 June 3;14(6):915-919.
The Roles of RAC1 and RAC1B in Colorectal Cancer and Their Potential Contribution to Cetuximab Resistance. Claudia Wahoski*, Bhuminder Singh*. Review. Cancers. 2024 Jul 6;16(13):2472.
Extracellular RNA in oncogenesis, metastasis, and drug resistance. Hannah Nelson, Jeffrey Franklin, Qi Liu, Alissa Weaver, Robert Coffey, et al. Review. RNA Biol. 2024 Jan;21(1):17-31.
Multiomic profiling of human clonal hematopoiesis reveals genotype and cell-specific inflammatory pathway activation. Heimlich JB, Bhat P, Silver AJ, Brown D, Savona MR, Bick AG, Ferrell PB, et al. Blood Adv. 2024 Jul 23;8(14):3665-3678.
Efficacy and Safety of Taletrectinib in Chinese Patients With ROS1+ Non-Small Cell Lung Cancer: The Phase II TRUST-I Study. Li W, Xiong A, Yu Q, Yan B, Lovly CM, Zhou C, et al. J Clin Oncol. 2024 Aug 1;42(22):2660-2670.
aKNNO: single-cell and spatial transcriptomics clustering with an optimized adaptive k-nearest neighbor graph. Li J, Shyr Y, Liu Q. Genome Biol. 2024 Aug 1;25(1):203.
Identification and multimodal characterization of a specialized epithelial cell type associated with Crohn’s disease. Jia Li*, Jennifer Pilat, Kara McNamara, Alain Gobert, Jeremy Goettel, Yash Choksi, David Schwartz, Yu Shyr, Keith Wilson*, Lori Coburn*, Ken Lau*, Qi Liu*, et al. Nature Communications. 2024 Aug 22;15(1):7204.

CONFERENCES OF INTEREST:
Society for Immunotherapy Cancer (SITC) Immuno-Oncology Drug Development Summit. September 30 – October 1, 2024, in Boston. Register here.
VCI 2024 Retreat. October 2-3, 2024. Fall Creek Falls, Spencer, TN. Contact summer.brown@vumc.org to register.
Society for Immunotherapy Cancer (SITC). November 6-10, 2024. Houston TX. Dr. Kim Rathmell will be preesenting. Register here.
American Association of Extracellular Vesicles – AAEV2024. November 10-13, 2024. Houston, TX. Register here.
American Society for Cell Biology (ASCB), EMBO. CellBio2024 conference December 14-18, 2024. San Diego, CA.
SEMINAR SERIES:
VICC Seminar series
VI4 Seminar Series To add the full 2024/2025 VI4 Seminar Series to your calendar, email: VI4Research@vumc.org.
|
|
The Program in Cancer Biology 2024-2025 Student Association Executive Board Members are busy organizing the 2024 Program in Cancer Biology Retreat.

Presidents: Elysa Pierro and KayLee Steiner. Philanthropy Chairs: Jared Rhodes, Sarah Ginther, and Logan Vlach. Social Chairs: Gwenyth Joseph and Jeremy Kane. Academic Chair: Emma Hathaway. Graduate Student Council Representative: Maxwell Hamilton.



Dominique Parker (Rhoades Lab) &
Matt Loberg (Weiss Lab)
We are proud to announce our Meharry-Vanderbilt-Tennessee State Cancer Partnership Equity in Cancer Research Program Award recipients! Be on the lookout for their cancer research presentations at a conference near you!
The 11th Annual Southeastern Immunology Symposium was hosted by the University of Alabama in Birmingham, August 16 – 17, 2024 at the Red Mountain Theatre. The symposium provides a forum to share the newest developments in basic, translational and clinical immunology to foster increased relationships and collaborations. Several leading faculty from institutions across the southeast presented their work. Vanderbilt University Faculty and Trainees were well represented at the conference.

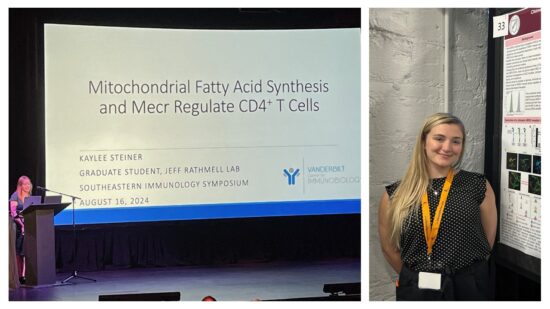
KayLee Steiner (Rathmell Lab) was chosen to give a presentation and won the AAI Young Investigator Oral Award at the Southeastern Immunology Symposium! KayLee discussed the Imbalanced effector and regulatory CD4 T cell subsets that can drive inflammatory diseases. These subsets rely on distinct metabolic programs, and metabolism modulation differentially affects T cell subsets. Because lipid metabolism is poorly understood in T cells, they performed targeted in vivo CRISPR/Cas9 screens to identify essential lipid metabolism genes and pathways. Multiple in vivo screens identified Mecr,Mcat and Oxsm of mitochondrial fatty acid synthesis (mtFAS) as important for T cell fitness. We focused on Mecr as it is rate-limiting and dynamically regulated in mtFAS, and variants cause a Mendelian inborn error in metabolism. Mecrfl/fl; Cd4cre KO mice had normal naïve CD4 and CD8 T cell numbers, demonstrating that Mecr is not acutely essential. However, effector and memory cells were reduced in the periphery, and Mecr was required for CD4 T cells to proliferate, differentiate, and survive when activated efficiently. Interestingly, Th1 cell differentiation was specifically increased upon acute activation, although T cells showed decreased mTORC1 activity and signs of mitochondrial stress in the absence of Mecr. Mecr-KO cells had decreased mitochondrial respiration, electron transport chain protein expression, and accumulated intracellular iron, which contributed to cell death and sensitivity to ferroptosis. Importantly, Mecr-deficient T cells had fitness disadvantages in in vivo inflammatory, tumor, and infection models. Thus, mtFAS and Mecr play important roles in activated T cells and may provide targets to modulate immunological function in inflammatory diseases. These findings also provide insight into the immunological state of Mecr- and other mtFAS-deficient patients.
Julia Steele (Balko Lab) presented her research at The 11th Annual Southeastern Immunology Symposium. Her poster presentation discussed the αHER2 antibody-drug conjugates (ADCs), which have transformed the treatment of HER2+ breast cancer, showing unprecedented success in the clinic. However, rigorous and representative preclinical models to study these therapeutics in vivo are lacking. This stems from the fact that trastuzumab, the antibody backbone of both FDA-approved αHER2 ADCs, does not bind to rodent HER2. Thus, most preclinical studies employ in vitro or immune-deficient (e.g., xenograft) models, limiting investigations of ADC function, immunologic effects, toxicity, and resistance. To address this issue, we developed a treatment-sensitive, chimerized mouse HER2 tumor model capable of binding to trastuzumab while avoiding growth suppression and immunologic selection in vivo, a limitation of existing models. Future studies will use this model to identify mechanisms of toxicity and resistance to αHER2 ADCs as well as to determine their effects and interactions with the tumor immune microenvironment.

Researchers from the School of Medicine Basic Sciences recently developed an analytical tool that could lead to the use of “liquid biopsies” as a substitute for traditional biopsies for certain patients or diseases. The tool, called EV Fingerprinting, was the culmination of the dissertation work of Ariana von Lersner, PhD, a former graduate student and current postdoctoral scholar in the lab of Alissa Weaver, MD, PhD, Cornelius Vanderbilt Professor of Cell and Developmental Biology. Read more here.

Vivian Weiss, MD, PhD, Associate Vice Chair for Clinical and Translational Research, Pathology, Microbiology, and Immunology Associate Professor Department of Pathology, Microbiology, and Immunology.
Dr. Weiss has accepted a promotion and will begin a secondary appointment in the Department of Cell and Developmental Biology. The Weiss lab is focused on understanding the molecular and immunologic mechanisms of thyroid cancer invasion and metastasis. While most thyroid cancer patients respond well to standard therapy, there are few options for those with widely metastatic or recurrent disease. Predicting which patients will have aggressive disease and treating those patients is an essential goal in the field. The laboratory aims to understand the signaling mechanisms and immune microenvironment responsible for the aggressive malignant behaviors of metastatic and recurrent disease. Projects include defining the role of Wnt signaling within the current molecular landscape of papillary thyroid carcinoma. The laboratory also uses computational immunogenomics to define the immunologic microenvironment within aggressive thyroid cancer subtypes to improve treatment and malignancy risk prediction at the time of biopsy.
 Sandy Zinkel, MD/PhD, Associate Professor of Medicine, Cancer Biology, Cell & Developmental Biology, Vanderbilt University School of Medicine, has been awarded the Clinician Scientist Investigator Award. This honor extends her Merit Award renewal for six more years. What an incredible accomplishment and recognition of Dr. Zinkel’s outstanding research in how normal and malignant cells regulate programmed cell death, critical knowledge for understanding cancer and developing new techniques to fight this devastating disease. The Zinkel lab is interested in the impact of programmed cell death mechanisms on the bone marrow microenvironment and progression to bone marrow failure and transformation to leukemia. As many cell death proteins reside in the mitochondria, we are also interested in how these cell death genes modulate mitochondrial structure and function and direct metabolic reprogramming during stress. Sandy Zinkel, MD/PhD, Associate Professor of Medicine, Cancer Biology, Cell & Developmental Biology, Vanderbilt University School of Medicine, has been awarded the Clinician Scientist Investigator Award. This honor extends her Merit Award renewal for six more years. What an incredible accomplishment and recognition of Dr. Zinkel’s outstanding research in how normal and malignant cells regulate programmed cell death, critical knowledge for understanding cancer and developing new techniques to fight this devastating disease. The Zinkel lab is interested in the impact of programmed cell death mechanisms on the bone marrow microenvironment and progression to bone marrow failure and transformation to leukemia. As many cell death proteins reside in the mitochondria, we are also interested in how these cell death genes modulate mitochondrial structure and function and direct metabolic reprogramming during stress.

Rebecca Ihrie, PhD
Associate Professor, Cell & Developmental Biology, and Neurological Surgery.
The School of Medicine Basic Sciences has won funding from university grants to propel early-stage research. Dr. Ihrie’s lab received one of the Spring 2024 Scaling Success grants for her research projects that have demonstrated potential and have received much interest from external sponsors. Dr. Ihrie and her lab team are focusing on populations of stem cells in the embryonic brain that are thought to be cells of origin for pediatric brain tumor growth through her funded project titled, “Mapping Preferential Translation in Neural Development.” Congratulations!
 Please enjoy this interview about the exciting research that The Rathmell lab is performing. Please enjoy this interview about the exciting research that The Rathmell lab is performing.
The Immunology Podcast interviewed Jeffrey Rathmell, Cornelius Vanderbilt Professor of Pathology, Microbiology and Immunology. Dr. Rathmell’s research focuses on T cell metabolism in cancer. He talks about what T cells use for fuel and his work on fatty acid synthesis. He also discusses the ‘obesity paradox’ in cancer immunotherapy and his favorite parts of being a scientist and professor.


Ambra Pozzi, PhD, Roy Zent, PhD
Dr’s Pozzi and Zent and their team recently published their exciting research in the Journal of Diabetes! They have discovered that the Cytochrome P450 epoxygenase Cyp2c44, a murine epoxyeicosatrienoic acid (EET)-producing enzyme, promotes insulin sensitivity, and Cyp2c44,-/- mice, show hepatic insulin resistance. Because insulin resistance leads to hepatic lipid accumulation and hyperlipidemia, we hypothesized that Cyp2c44 regulates hepatic lipid metabolism. Standard chow diet (SCD)-fed male Cyp2c44-/- mice had significantly decreased EET levels and increased hepatic and plasma lipid levels compared with wild-type mice. We showed increased hepatic plasma membrane localization of the FA transporter 2 (FATP2) and total unsaturated fatty acids and diacylglycerol (DAG) levels. Cyp2c44-/- mice had impaired glucose tolerance and increased hepatic plasma membrane-associated PKCδ and phosphorylated IRS-1, two negative regulators of insulin signaling. Surprisingly, SCD and high-fat diet (HFD)-fed Cyp2c44-/- mice had similar glucose tolerance and hepatic plasma membrane PKCδ levels, suggesting that SCD-fed Cyp2c44-/- mice have reached their maximal glucose intolerance. Inhibition of PKCδ decreased IRS-1 serine phosphorylation and improved insulin-mediated signaling in Cyp2c44-/- hepatocytes. Finally, Cyp2c44-/- HFD-fed mice treated with the analog EET-A showed decreased hepatic plasma membrane FATP2 and PCKδ levels with improved glucose tolerance and insulin signaling. In conclusion, loss of Cyp2c44 with concomitantly decreased EET levels leads to increased hepatic FATP2 plasma membrane localization, DAG accumulation, and PKCδ-mediated attenuation of insulin signaling. Thus, Cyp2c44 acts as a regulator of lipid metabolism by linking it to insulin signaling. Read more here.

Through support from Vanderbilt-Ingram Cancer Center, all Vanderbilt University and Vanderbilt University Medical Center faculty, staff, and trainees have paid access to the Nature Masterclasses online training courses. These bite-sized online courses are specifically designed to be used on demand and at your rhythm. There are subjects and courses covering a wide range of topics for any career stage.
Current course offerings:
Writing a Research Paper
Publishing a Research Paper
Writing and Publishing a Review Paper
Research Integrity: Publication Ethics
Focus on Peer Review
Experiments: From Idea to Design
Persuasive Grant Writing
Finding Funding Opportunities
Managing Research Data to Unlock its Full Potential
Data Analysis: Planning and Preparing
Data Analysis: Conducting and Troubleshooting
Interpreting Scientific Results
Narrative Tools for Researchers
Effective Science Communication
Advancing Your Scientific Presentations
Creating Successful Research Posters
Getting an Academic Research Position
Networking for Researchers
Introduction to Collaboration
Participating in a Collaboration
Leading a Collaboration
For additional information, contact Caroline Hartford at caroline.hartford@vumc.org.
 Have you received an award, a paper published or any other good news you would like to celebrate with our community? If so, please e-mail: kerry.w.vazquez@vanderbilt.edu. Have you received an award, a paper published or any other good news you would like to celebrate with our community? If so, please e-mail: kerry.w.vazquez@vanderbilt.edu.
Newsletter header photo credit to Dr. Anna Vilgelm, “DNA Comets.” Articles and Pictures credit to VU and VUMC. All other photo credit to unsplash.
|
|
|
Vanderbilt University is committed to principles of equal opportunity and affirmative action.
Vanderbilt®, Vanderbilt University®, V Oak Leaf Design®, Star V Design® and Anchor Down® are trademarks of The Vanderbilt University. ©2025 Vanderbilt University. All rights reserved.
|
|
![Medicine - Cancer Biology E-Newsletter [Vanderbilt University]](https://cdn.vanderbilt.edu/vu-URL/wp-content/uploads/sites/119/2017/11/19151037/cancer-biology-design-004.png)