A glimpse into higher-order mRNP assemblies
 In eukaryotic cells, mRNA is transcribed in the nucleus. Protein factors rapidly bind to newly transcribed mRNA to form a messenger ribonucleoprotein (mRNP), which is exported to the cytoplasm for translation.
In eukaryotic cells, mRNA is transcribed in the nucleus. Protein factors rapidly bind to newly transcribed mRNA to form a messenger ribonucleoprotein (mRNP), which is exported to the cytoplasm for translation.
One complex in humans vital for the formation and export of mRNPs is the transcription-export complex (TREX). TREX consists of the THO complex, an export adaptor protein ALYREF, and DEAD-box ATPase DDX39B. DDX39B has also been shown to interact with SARNP, a ribonucleoprotein implicated in mRNA export. However, it has been unclear how these proteins assemble into a higher-order complex and facilitate mRNP formation.
In this recent study from the Ren group, Yihu Xie et al. use a combination of structural and functional studies to investigate the assembly and function of DDX39B and SARNP. A crystal structure was determined of DDX39B, Tho1 (yeast ortholog of SARNP), and RNA at 2.5 Å resolution. Tho1 was found to interact with two DDX39B proteins simultaneously through two evolutionarily conserved DDX39B interacting motifs (DIMs). Interestingly, different species contain different numbers of DIMs within their SARNP orthologs which correlate with the complexity of that species genome.
DDX39B was also shown to bind RNA through conserved motifs. Additionally, RNA assembles on the same side of both DDX39B proteins, 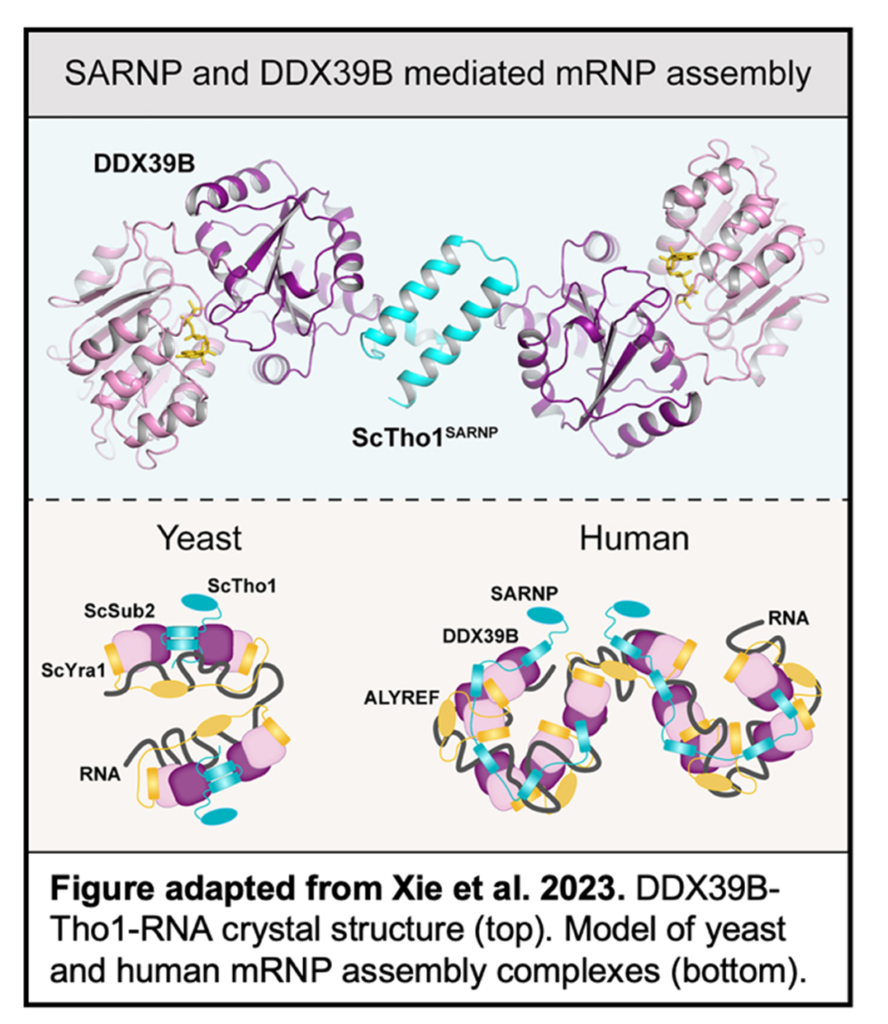 implying that a longer strand could be bound by two DDX39B proteins simultaneously. To determine the functional implications of the SARNP-DDX39B-RNA complex, siRNA knockdowns were conducted in A549 cells. RNA-seq of the nuclear and cytosolic fractions of these cells determined a select subset of mRNAs exhibit impaired export, specifically those with a high GC content.
implying that a longer strand could be bound by two DDX39B proteins simultaneously. To determine the functional implications of the SARNP-DDX39B-RNA complex, siRNA knockdowns were conducted in A549 cells. RNA-seq of the nuclear and cytosolic fractions of these cells determined a select subset of mRNAs exhibit impaired export, specifically those with a high GC content.
Overall, this study determined key structural and functional traits about the SARNP-DDX39B-RNA complex, providing vital information for our understanding of RNA export from the nucleus and the role of higher-order assembly in mRNP formation.
Read more here. ~ Cameron I. Cohen
Leave a Response
You must be logged in to post a comment