From activation to repression: decoding the impact of PPARγ ligands on ligand-binding domain conformation
 Nuclear receptors (NRs) are a group of transcription factors that control gene expression in response to endogenous metabolites and synthetic ligands. Ligands bind the C-terminal ligand-binding domain (LBD) of NRs which is widely believed to alternate between transcriptionally active and repressive states and be stabilized upon ligand binding. Various X-ray crystallography and cryo-electron microscopy studies have determined the structures of NRs bound to ligands, coregulators, and DNA, but these methods fail to capture the dynamic conformational shifts of the LBD. Further, previous work has largely focused on compounds of a single pharmacological type, such as agonists, instead of focusing on the full pharmacological spectrum.
Nuclear receptors (NRs) are a group of transcription factors that control gene expression in response to endogenous metabolites and synthetic ligands. Ligands bind the C-terminal ligand-binding domain (LBD) of NRs which is widely believed to alternate between transcriptionally active and repressive states and be stabilized upon ligand binding. Various X-ray crystallography and cryo-electron microscopy studies have determined the structures of NRs bound to ligands, coregulators, and DNA, but these methods fail to capture the dynamic conformational shifts of the LBD. Further, previous work has largely focused on compounds of a single pharmacological type, such as agonists, instead of focusing on the full pharmacological spectrum.
In this study from the Doug Kojetin and John Yang labs, peroxisome proliferator-activated receptor gamma (PPARγ) is used as a model system to understand the functional shifts of nuclear receptor LBDs.
The researchers first designed a series of inverse agonists using 2-chloro-5-nitrobenzamide as a scaffold and solely altering the amine R group. Previous structures have shown that in the transcriptionally active conformation, helix 12 of PPARγ is solvent exposed and facilitates coactivator binding. Conversely, in the repressive conformation, helix 12 occupies the orthosteric ligand-binding pocket and leaves surface regions exposed for corepressor binding. Additionally, a pi-stacking interaction is observed in the repressive structure between the inverse agonist and residues in PPARγ to form an aromatic triad.
With this knowledge in mind, potential inverse agonists were identified from the ZINC database or synthesized. A combination of time-resolved fluorescence resonance energy transfer corepressor peptide interaction assay and a cell-based transcriptional reporter assay determined that each of the identified compounds displayed repressive activity. The authors then characterized a ligand series of twenty 2-chloro-5-nitrobenzamide analogs and found the compounds spanned a 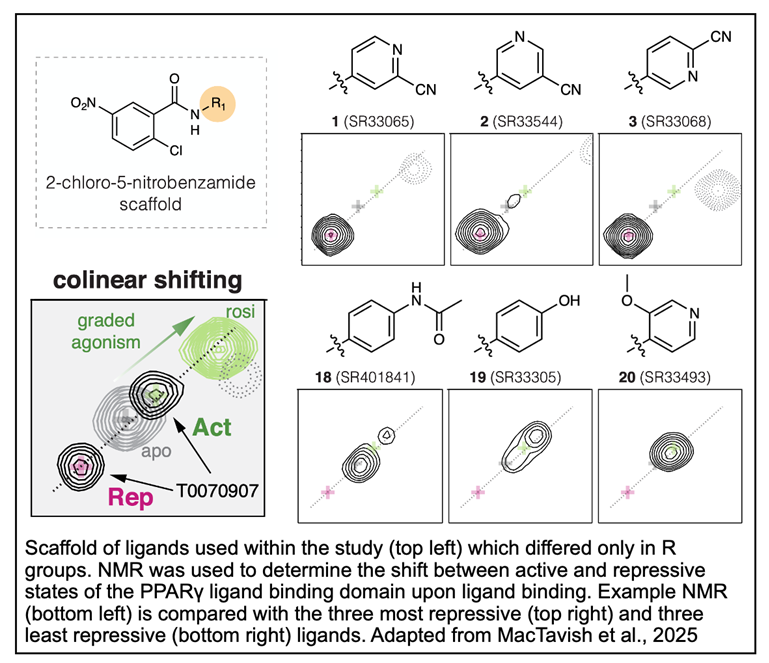 pharmacological range from inverse agonist to neutral antagonist to agonist. Interestingly, all inverse agonists contained a polar pyridyl aromatic ring while most agonists had a hydrophobic non-polar phenyl ring.
pharmacological range from inverse agonist to neutral antagonist to agonist. Interestingly, all inverse agonists contained a polar pyridyl aromatic ring while most agonists had a hydrophobic non-polar phenyl ring.
In order to more specifically ascertain the structural basis of inverse agonism, crystal structures were determined of PPARγ bound to the most effectively repressive compounds. In all of the structures, helix 12 of PPARγ adopted the solvent-occluded conformation and the aromatic triad residues interacted with the inverse agonist R groups. Finally, nuclear magnetic resonance (NMR) spectroscopy was utilized to determine the effect of inverse agonists on PPARγ dynamics. The inverse agonists were all found to shift the LBD of PPARγ towards a repressive conformation and excitingly, there was a strong correlation between a compound’s repressive activity and the strength of the repressive NMR peak in PPARγ.
Overall, this study has not only identified key structural features of inverse agonism, constructed a more comprehensive ligand series for future studies, but also provided key insights into the functional regulation of NRs. Future studies, and in particular drug design studies, will benefit greatly from a focus on the dynamic nature of PPARγ.
Be sure to check out the full story of NR regulation in Nature Communications! ~ Cameron I. Cohen

Leave a Response
You must be logged in to post a comment