Understanding NEIL1-RPA binding at the intersection of DNA repair and replication
 Reactive oxygen species damage DNA through the oxidation of bases and can result in a variety of diseases such as cancer, accelerated aging, and neurodegeneration. Single-strand DNA, which exists transiently during replication, is especially sensitive to reactive oxygen species. Oxidized bases are readily repaired by the base excision repair (BER) pathway, but incomplete repair during replication can lead to highly toxic strand breaks. Therefore, tight coordination between BER and replication machinery is required to maintain genome stability in replicating cells.
Reactive oxygen species damage DNA through the oxidation of bases and can result in a variety of diseases such as cancer, accelerated aging, and neurodegeneration. Single-strand DNA, which exists transiently during replication, is especially sensitive to reactive oxygen species. Oxidized bases are readily repaired by the base excision repair (BER) pathway, but incomplete repair during replication can lead to highly toxic strand breaks. Therefore, tight coordination between BER and replication machinery is required to maintain genome stability in replicating cells.
NEIL1 is a DNA glycosylase which initiates BER of oxidized bases and is critical for pre-replicative repair. NEIL1 is upregulated during S-phase and is known to interact with replication enzymes in addition to BER machinery. One such example, RPA, is a replication enzyme which modulates NEIL1 activity differently on single-strand DNA and double-strand DNA to prevent strand breaks. The interplay between NEIL1 and RPA is therefore crucial to understand BER activity during replication, but the molecular forces mediating this interaction are not well characterized.
In this study from Rémy Le Meur, former postdoc in the Chazin lab, a combination of nuclear magnetic resonance spectroscopy (NMR), isothermal titration calorimetry (ITC), computational modelling, and cell-based techniques are used to interrogate the relationship between NEIL1 and RPA.
The authors first sought to identify the specific location of binding on RPA and NEIL1 using two-dimensional 15N-1H Heteronuclear Single Quantum Coherence (HSQC) NMR. Three domain fragments from the RPA trimer were generated based on previous work, specifically the two protein recruitment domains RPA70N and RPA32C, along with the tandem high affinity DNA binding domains RPA70AB. Line broadening of RPA domain signals indicated that NEIL1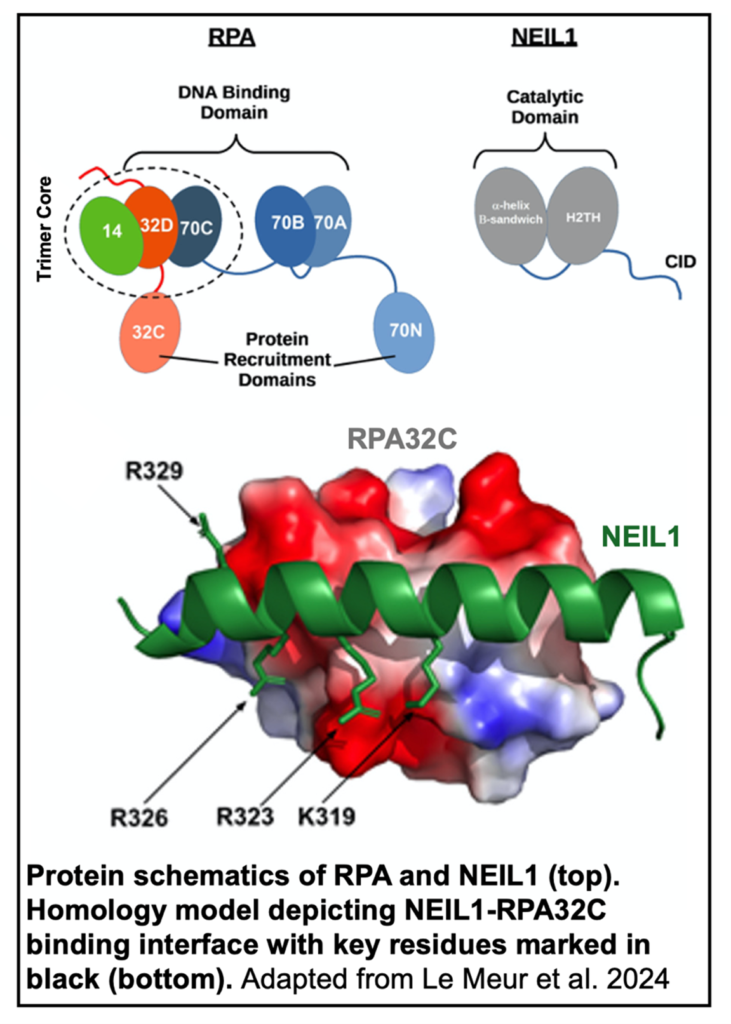 binds RPA32C, and to a lesser extent RPA70AB. ITC further revealed that NEIL1 bound RPA32C with a dissociation constant of 240 nM.
binds RPA32C, and to a lesser extent RPA70AB. ITC further revealed that NEIL1 bound RPA32C with a dissociation constant of 240 nM.
Binding to RPA32C was mapped to a specific motif in the common interaction domain of NEIL1 using a multiple sequence alignment of previously characterized RPA32C motifs. A homology model of the NEIL1 RPA-binding motif and RPA32C was then generated using previously published co-crystal structures. Identification of key interacting residues from the model allowed the authors to design a multi-site charge reversal NEIL1 mutant which exhibited substantially decreased binding to RPA.
Finally, the authors sought to investigate the role of NEIL1-RPA binding within the context of DNA repair. Interestingly, mutations in the RPA-binding motif of NEIL1 did not inhibit rates of DNA lesion repair in an in vitro biochemical assay. Further, U2OS cells with mutant NEIL1 exhibited a decrease in nucleotide excision repair, a separate DNA repair pathway.
Overall, this paper identified key molecular details of NEIL1-RPA binding and leveraged this knowledge to design an RPA-binding NEIL1 mutant for further experiments. The authors’ in vitro and cell data also raise interesting questions about the role of NEIL1-RPA binding within NER, paving the way for future research into DNA repair pathway crosstalk.
Read more about this study in JBC! ~ Cameron I.Cohen
Leave a Response
You must be logged in to post a comment