Hitting A. baumannii where it hurts: novel insights into DNA repair pathways as therapeutic targets
 Acinetobacter baumannii is a hospital-acquired human pathogen that can result in serious infections of the blood, wounds, lungs, and urinary tract. These infections are further complicated by rapid evolution of antibiotic resistance in this organism, making A. baumannii the fifth leading cause of antimicrobial resistance-associated deaths globally. There is, therefore, an urgent need for novel therapeutics against multidrug-resistant A. baumannii. Additionally, as this pathogen lacks classic pharmacological targets, such as toxins, identifying alternate drug targets is a critical first step.
Acinetobacter baumannii is a hospital-acquired human pathogen that can result in serious infections of the blood, wounds, lungs, and urinary tract. These infections are further complicated by rapid evolution of antibiotic resistance in this organism, making A. baumannii the fifth leading cause of antimicrobial resistance-associated deaths globally. There is, therefore, an urgent need for novel therapeutics against multidrug-resistant A. baumannii. Additionally, as this pathogen lacks classic pharmacological targets, such as toxins, identifying alternate drug targets is a critical first step.
During infection of a host, bacterial pathogens encounter a wide variety of genotoxic agents, or compounds which damage DNA, such as reactive oxygen species, reactive nitrogen species, acidic pH, antibiotics, and hypochlorous acid which damage DNA. One example of damage is interstrand DNA crosslinks (ICLs) in which covalent linkages are formed between opposing strands of DNA. ICLs are particularly toxic as this type of DNA damage interferes with replication and transcription. Bacterial genomes, therefore, maintain a large number of DNA repair enzymes, including DNA glycosylases which repair ICLs.
This work, conducted by postdoctoral researchers Dillon Kunkle and Yujuan Cai from the Skaar lab and Eichman lab respectively, focuses on characterizing a newly discovered DNA glycosylase, renamed AlkX, in A. baumannii. Using a combination of in vitro and in vivo methods, the authors determine the functionality of AlkX and demonstrate its viability as a novel drug target for A. baumannii therapeutics.
In order to characterize the specificity of AlkX repair activity, Kunkle & Cai et al. treated A. baumannii with a variety of DNA damaging agents. Expression of the alkX gene only increased in response to nitrogen mustard (NM) mechlorethamine, a DNA damaging agent which causes ICLs. Further, growth of A. baumannii lacking alkX was reduced in the presence of mechlorethamine, but not other genotoxic compounds. In vitro, purified AlkX protein was shown to exhibit ICL unhooking 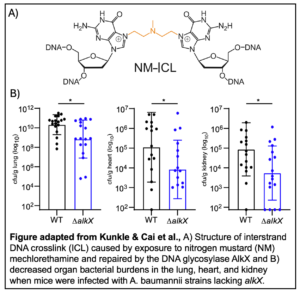 activity in response to NM treatment.
activity in response to NM treatment.
The authors also demonstrated that maintenance of genome integrity, specifically by AlkX, plays a role in A. baumannii virulence. In mouse infection models, A. baumannii strains lacking AlkX exhibited decreased colonization of the lung, heart, and kidney tissues.
Finally, as NM is not typically found in biological settings, it was important to determine the physiological agent inducing AlkX activity during infection. As lung tissue sustains pH levels below 5 and is frequently colonized by A. baumannii, acidic pH was explored as a signal for AlkX. Subsequently, when A. baumannii was grown at pH 5.25, alkX expression increased and strains lacking AlkX exhibited decreased fitness.
Overall, this work has demonstrated that AlkX activity is important not only for inherent genome stability, but also during infection, allowing bacterial DNA to hold up under the genotoxic stress of low pH.
Read more about this exciting prospect for drug development in PNAS! ~ Cameron I. Cohen

Leave a Response
You must be logged in to post a comment