On the origins of pentose sugars in everninomicin D biosynthesis
Everninomicins are a family of naturally produced oligosaccharides, some of which exhibit ribosome-targeting antibiotic activity. Everninomicin A (EVA) was developed into late-phase clinical trials as an antibiotic but eventually pulled due to toxicity concerns. Toxicity was attributed to aggregates in the formulations, likely caused by π-stacking interactions from flanking aromatic rings in EVA. Current research is focused on an equipotent analog of EVA, everninomicin D (EVD), which has a single aromatic ring and is precluded from π-stacking concerns, making it a promising clinical candidate.
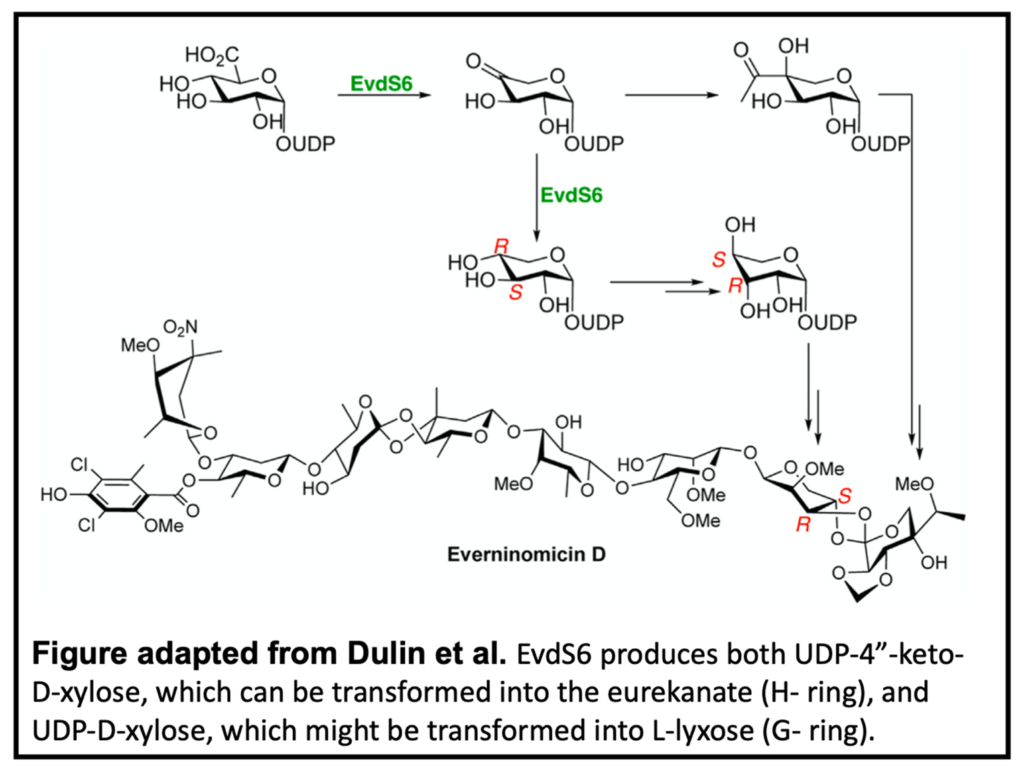 The biosynthesis of EVD, however, is poorly understood. In particular, the biosynthesis of the two terminating pentose sugars in place of one aromatic ring in EVA are integral to understanding the entire molecule. In this study, Dr. Callie Dulin, a recent graduate of the Bachmann research group, works in collaboration with the Iverson lab, to characterize a novel glucuronic acid decarboxylase.
The biosynthesis of EVD, however, is poorly understood. In particular, the biosynthesis of the two terminating pentose sugars in place of one aromatic ring in EVA are integral to understanding the entire molecule. In this study, Dr. Callie Dulin, a recent graduate of the Bachmann research group, works in collaboration with the Iverson lab, to characterize a novel glucuronic acid decarboxylase.
Sequence analysis identified EvdS6, which falls into a family of enzymes known for producing pentose sugars. NMR analysis revealed that unlike most decarboxylases, EvdS6 produces two products, oxidized UDP-4”-keto-D-xylose and reduced UDP-D-xylose. This bifunctional nature allows EvdS6 to potentially produce sugar precursors for both pentose-derived sugars of the EVD scaffold, the H- and G-rings respectively. X-ray crystallography and selective mutagenesis were also used to determine the active site architecture of EvdS6 and the critical residues for reduction.
While future research is required to fully ascertain the role EvdS6 plays in EVD biosynthesis, this study defines the likely origins of the EVD pentose sugars.
Read more here! ~Cameron I. Cohen

Leave a Response
You must be logged in to post a comment