Structural plasticity in bacterial flagellar motors
 Chemotaxis is the process of moving in response to one’s environment, and in bacteria, this process is supported largely by flagella. The flagellum is a motility organelle comprised of an elongated filament and various rings, consistent with its role as a rotary motor. One component, the MS-ring, is vital, both for the assembly and rotation of the bacterial filament. The MS-ring is made up of repeating subunits of the membrane-spanning protein FliF, but the number of protomers within the structure has been a subject of debate. Further, the FliF proteins contain multiple domains, including a group of ring-building motifs (RBMs). The stoichiometry and position of the RBMs within the MS-ring have been unclear.
Chemotaxis is the process of moving in response to one’s environment, and in bacteria, this process is supported largely by flagella. The flagellum is a motility organelle comprised of an elongated filament and various rings, consistent with its role as a rotary motor. One component, the MS-ring, is vital, both for the assembly and rotation of the bacterial filament. The MS-ring is made up of repeating subunits of the membrane-spanning protein FliF, but the number of protomers within the structure has been a subject of debate. Further, the FliF proteins contain multiple domains, including a group of ring-building motifs (RBMs). The stoichiometry and position of the RBMs within the MS-ring have been unclear.
Prash Singh, senior research associate in the Iverson lab, recently led a study aimed at discerning the organization of the MS-ring in Salmonella enterica flagella. With help from collaborator Terunaga Nakagawa, Singh used Cryo-EM to determine high-resolution structures of the FliF subunits within the MS-ring structure. FliF was co-expressed with FliG, FliM and FliN, 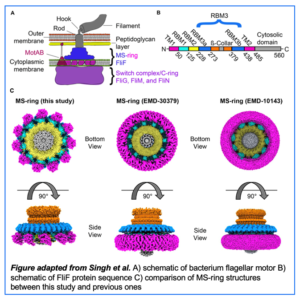 proteins which make up the switch complex of the flagellum, to produce a stable “post-assembly” complex. 2D class averages in this study show post-assembly MS-rings can contain 32, 33 or 34 FliF subunits. The RBMs within these FliF proteins also display variable, distinct symmetries.
proteins which make up the switch complex of the flagellum, to produce a stable “post-assembly” complex. 2D class averages in this study show post-assembly MS-rings can contain 32, 33 or 34 FliF subunits. The RBMs within these FliF proteins also display variable, distinct symmetries.
The authors propose that different FliF subunit numbers could allow for stepwise increases in torque when responding to increases in load. Further, the structural plasticity at multiple levels of flagellar organization could support the ability of bacteria to react to changes in the environment.
Read more about the structures here! ~ Cameron I. Cohen

Leave a Response
You must be logged in to post a comment