Protein Trafficking: An interactive network of coats, cargos and adaptors – Oh my!
Come take a ride along the intracellular highways that are trafficking pathways!
Cells use vesicles to transport diverse cargo, such as transmembrane proteins, between cellular compartments.
In this complex system, membrane coat proteins work together with adaptor proteins to recruit cargo, encase them within vesicles and deliver them to the proper destination. These coat proteins are highly conserved in eukaryotes, and their dysregulation leads to diseases such as microcephaly and cancer.
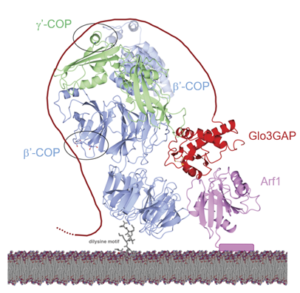
While clathrin is probably the most well studied coat protein, COPI and COPII (and their associated proteins) have been the recent focus of Lauren Jackon’s lab. Betty Xie, Amy Kendall and Lauren Jackson recently published an article in the Journal of Cell Biology detailing how the b’-COPI subunit interacts with Glo3, the GTPase-Activating Protein for Arf1.
By combining elegant biochemical and biophysical techniques, they delineated the exact regions of interaction between
b’-COPI and Glo3. Furthermore, they tested these interactions by introducing point mutations which decreased binding affinity in vitro, and caused mis-localization of specific cargo in yeast. Overall, this thorough study brings the field one step closer to understanding how COPI interacts with its partners to ensure cargo reaches its final destination.
Read more about these findings!
Leave a Response
You must be logged in to post a comment