Example-CH4
1. Sum the Valence electrons for CH4 . The number of valence electrons corresponds to the element's group number.
C = 4 = 4
H = 1 (x4) = 4
8 Valence electrons
2. Using a pair of electrons per bond, draw in the four C--H single bonds:
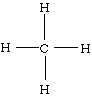
3. Distribute the remaining electrons to achieve a noble gas electron configuration for each atom. Since eight electrons have been used forming the four bonds, no electrons remain to be distributed. Since there are no more electrons remaining, our structure is complete.
![]()